Can Modafinil Cause Stevens-Johnson Syndrome?
Modafinil is a wakefulness-promoting medicine used for narcolepsy, obstructive sleep apnea, and shift work disorder. It is generally well tolerated. Rarely, a serious skin reaction called Stevens–Johnson syndrome (SJS) has been reported with modafinil and with its R-enantiomer, armodafinil. Knowing the early signs and what to do next helps reduce harm (U.S. Food and Drug Administration, 2015).
What is Stevens–Johnson syndrome (SJS)?
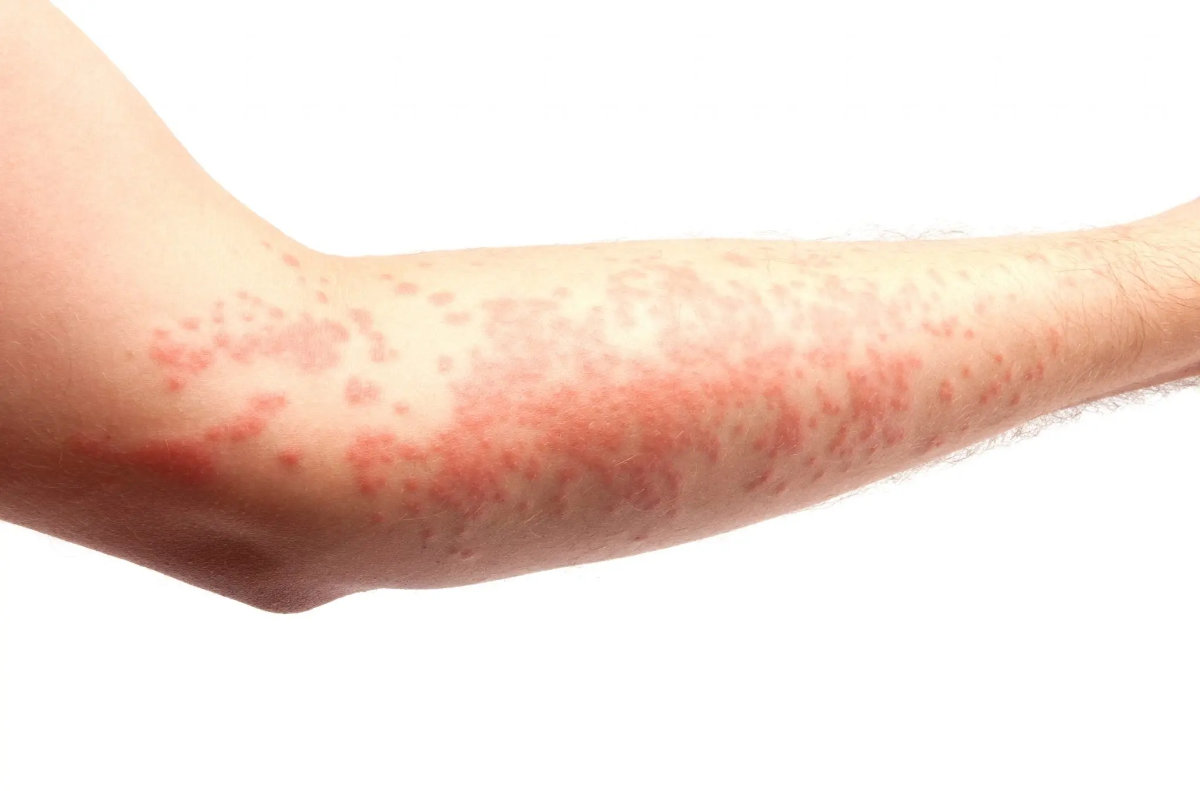
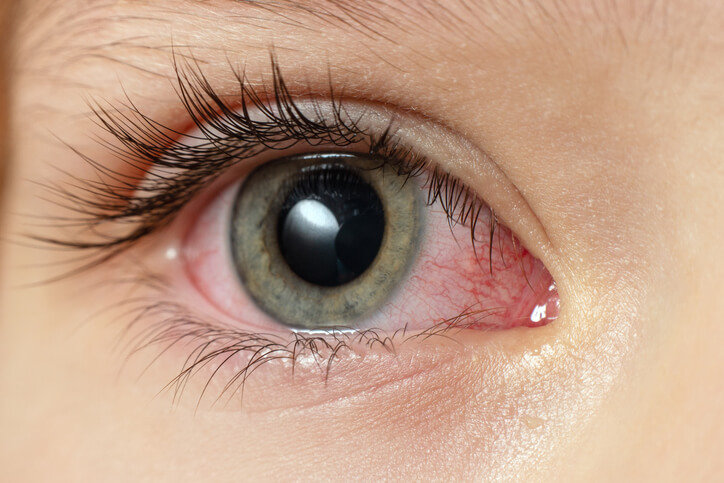
Stevens–Johnson syndrome is a severe reaction that causes the top layer of skin and mucous membranes to die and peel. Most cases are triggered by medicines. Symptoms usually start with fever, sore throat, cough, or eye irritation for a few days, then a painful rash, blisters, and raw skin appear. The mouth, eyes, and genitals are commonly involved (Oakley & Krishnamurthy, 2023; Hazin et al., 2008).
SJS is part of a spectrum with toxic epidermal necrolysis. Doctors classify severity by the percent of body surface area that peels. Less than 10 percent is SJS, 10 to 30 percent is overlap, more than 30 percent is toxic epidermal necrolysis (Oakley & Krishnamurthy, 2023).
Is SJS linked to modafinil or armodafinil?
Yes, rare cases have been reported in medical literature and postmarketing safety reports. A biopsy-confirmed case occurred about three weeks after starting high-dose modafinil in a 40-year-old woman, with mouth and genital erosions and a target-like rash that healed after treatment (Prince et al., 2018). A biopsy-confirmed case also occurred 12 days after starting armodafinil in a 21-year-old woman, with fever, lymph node swelling, and a typical SJS rash that improved after stopping the drug and receiving supportive care (Holfinger et al., 2018).
These reports show a temporal association. They do not prove causation for every patient, but they support the safety warnings found in product labeling (U.S. Food and Drug Administration, 2015).
What does the U.S. Food and Drug Administration advise?
The U.S. Food and Drug Administration labeling for modafinil states that serious rash, including SJS and toxic epidermal necrolysis, has been reported. The label instructs patients and clinicians to discontinue modafinil at the first sign of rash unless the rash is clearly not drug related. The label also notes rare reports of drug rash with eosinophilia and systemic symptoms and advises caution regarding hypersensitivity reactions. Modafinil is not approved for pediatric use because of rash risk seen in trials and postmarketing reports (U.S. Food and Drug Administration, 2015).
How common is SJS in general?
SJS and toxic epidermal necrolysis together affect only a few people per million each year in the general population. Most cases are linked to medicines. In many patients, symptoms develop within one to eight weeks after starting the culprit drug. Some patients are at higher risk because of certain infections or genetic factors, depending on the medicine involved (Oakley & Krishnamurthy, 2023; Frantz et al., 2021).
Precise incidence of SJS with modafinil is unknown. Evidence comes mainly from case reports and postmarketing surveillance, which capture very rare events but cannot give exact risk (U.S. Food and Drug Administration, 2015; Greenblatt & Adams, 2023).
What are the early signs to watch for?
Call your clinician urgently and stop the medicine if you notice any of the following soon after starting modafinil or armodafinil, especially in the first six weeks (U.S. Food and Drug Administration, 2015; Greenblatt & Adams, 2023; Oakley & Krishnamurthy, 2023):
- Fever, sore throat, cough, red eyes
- Painful spreading rash, target-like spots, blisters
- Sores in the mouth, eyes, or genitals
- Skin pain or areas that peel
What happens in the body during SJS?
SJS is an immune reaction. Drug-specific T cells appear to attack skin cells. Signals such as granulysin, Fas ligand, and tumor necrosis factor trigger skin cell death. These pathways explain the rapid blistering and peeling seen in SJS and toxic epidermal necrolysis (Oakley & Krishnamurthy, 2023; Frantz et al., 2021).
How is SJS treated?
Treatment should start in a hospital, often in a burn unit or intensive care unit if the skin loss is extensive. Key steps include stopping the culprit drug, careful wound care, fluids, pain control, nutrition, and early involvement of specialists such as dermatology and ophthalmology. Doctors may calculate a severity score to estimate risk. Some centers consider systemic therapies such as corticosteroids, intravenous immunoglobulin, cyclosporine, or etanercept. Evidence varies by setting, and supportive care remains the foundation of treatment (Frantz et al., 2021; Hazin et al., 2008).
Practical guidance for patients and clinicians
- If a rash appears while taking modafinil or armodafinil, stop the drug and contact a clinician immediately. Do not restart unless a clinician finds a clear non-drug cause (U.S. Food and Drug Administration, 2015).
- After a serious rash, avoid rechallenge with modafinil or armodafinil. Discuss alternatives with your clinician (U.S. Food and Drug Administration, 2015; Greenblatt & Adams, 2023).
- Children should not take modafinil. The medicine is not approved for pediatric use because of serious rash risk in trials and reports (U.S. Food and Drug Administration, 2015).
Evidence highlights
- Modafinil case report with biopsy confirmation and three-week latency, full recovery after treatment (Prince et al., 2018).
- Armodafinil case report with biopsy confirmation and 12-day latency, improvement after stopping the drug (Holfinger et al., 2018).
- Labeling instructs discontinuation at first sign of rash, notes rare but serious reactions, and restricts pediatric use (U.S. Food and Drug Administration, 2015).
- Clinical overviews describe typical timing, symptoms, mechanisms, and current management approaches (Oakley & Krishnamurthy, 2023; Frantz et al., 2021; Hazin et al., 2008; Greenblatt & Adams, 2023).
FAQs
Does the risk of SJS depend on the dose of modafinil?
There is not enough evidence to show a dose–response risk. Published cases occurred within usual clinical dose ranges as well as at higher doses. The key factor is recent drug start, often within one to eight weeks (Prince et al., 2018; Oakley & Krishnamurthy, 2023).
Can I restart modafinil after a rash goes away?
Do not restart after a serious rash or suspected SJS. Discuss other options with your clinician. Cross-use with armodafinil is also avoided after a serious rash because the drugs are closely related (U.S. Food and Drug Administration, 2015; Greenblatt & Adams, 2023).
What should I do if I get mouth sores or eye redness while on modafinil?
Stop the medicine and seek urgent medical care. Mouth, eye, or genital sores with a rash can be early SJS signs and need prompt evaluation (U.S. Food and Drug Administration, 2015; Oakley & Krishnamurthy, 2023).
Is modafinil safe for children?
Modafinil is not approved for pediatric use. Serious rashes, including SJS, were seen in pediatric trials and postmarketing reports (U.S. Food and Drug Administration, 2015).
References
- U.S. Food and Drug Administration. (2015). PROVIGIL® (modafinil) tablets, for oral use, C-IV [Prescribing information]. U.S. Department of Health and Human Services. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020717s037s038lbl.pdf
- Greenblatt, K., & Adams, N. (2023). Modafinil. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK531476/
- Frantz, R., Huang, S., Are, A., & Motaparthi, K. (2021). Stevens–Johnson syndrome and toxic epidermal necrolysis: A review of diagnosis and management. Medicina, 57(9), 895. https://doi.org/10.3390/medicina57090895
- Hazin, R., Ibrahimi, O. A., Hazin, M. I., & Kimyai‐Asadi, A. (2008). Stevens–Johnson syndrome: Pathogenesis, diagnosis, and management. Annals of Medicine, 40(2), 129–138. https://doi.org/10.1080/07853890701753664
- Holfinger, S., Roy, A., & Schmidt, M. (2018). Stevens–Johnson syndrome after armodafinil use. Journal of Clinical Sleep Medicine, 14(5), 885–887. https://doi.org/10.5664/jcsm.7132
- Oakley, A. M., & Krishnamurthy, K. (2023). Stevens–Johnson syndrome. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459323/
- Prince, V., Philippidou, M., Walsh, S., & Creamer, D. (2018). Stevens–Johnson syndrome induced by modafinil. Clinical and Experimental Dermatology, 43(2), 191–192. https://doi.org/10.1111/ced.13282