Modafinil for Sleep Apnea: Efficacy, Dosage, and Considerations
Sleep apnea is a common disorder marked by repeated breathing interruptions during sleep. These disruptions cause excessive daytime sleepiness, severely impacting quality of life. Modafinil, a wake-promoting agent, has been studied as an adjunctive treatment for managing this sleepiness.
Understanding Sleep Apnea
Sleep apnea involves repeated pauses in breathing during sleep. The main types are:
- Obstructive Sleep Apnea (OSA): caused by physical airway collapse.
- Central Sleep Apnea (CSA): caused by failure of respiratory effort.
OSA affects approximately 2–4% of middle-aged adults and is often undiagnosed (Schwartz et al., 2003). It results in fragmented sleep, excessive daytime sleepiness, cognitive decline, and increased cardiovascular risk (Chapman et al., 2016).
The standard treatment is continuous positive airway pressure (CPAP) therapy, which stabilizes the airway and reduces apneic events. Nonetheless, some patients continue to experience residual sleepiness despite adherence to CPAP (Schwartz et al., 2003; Chapman et al., 2016).
Modafinil’s Role in Managing Sleep Apnea-Related Sleepiness
Modafinil is a novel wakefulness-promoting drug that differs pharmacologically from traditional stimulants. It primarily inhibits dopamine reuptake and influences orexin, histamine, and glutamate systems, contributing to enhanced alertness without typical stimulant side effects (Greenblatt & Adams, 2023).
Efficacy in Residual Sleepiness Despite CPAP
Clinical trials have shown that modafinil significantly reduces residual daytime sleepiness in OSA patients using CPAP (Schwartz et al., 2003; Chapman et al., 2016). A 12-week open-label study demonstrated sustained improvements in ESS scores and quality of life with modafinil dosed between 200 to 400 mg daily (Schwartz et al., 2003).
Efficacy in Untreated Mild to Moderate OSA
Modafinil has also been shown to improve sleepiness in patients with mild to moderate OSA not using standard treatments. A randomized placebo-controlled crossover trial showed that 200 mg daily modafinil significantly improved subjective sleepiness and psychomotor vigilance after two weeks (Chapman et al., 2014).
Meta-Analysis Summary
A meta-analysis of 10 randomized controlled trials including 1,466 patients concluded that modafinil and armodafinil improve both subjective (ESS) and objective (Maintenance of Wakefulness Test) sleepiness measures. However, modafinil treatment is associated with increased adverse events and treatment discontinuations, necessitating careful risk-benefit analysis (Chapman et al., 2016).
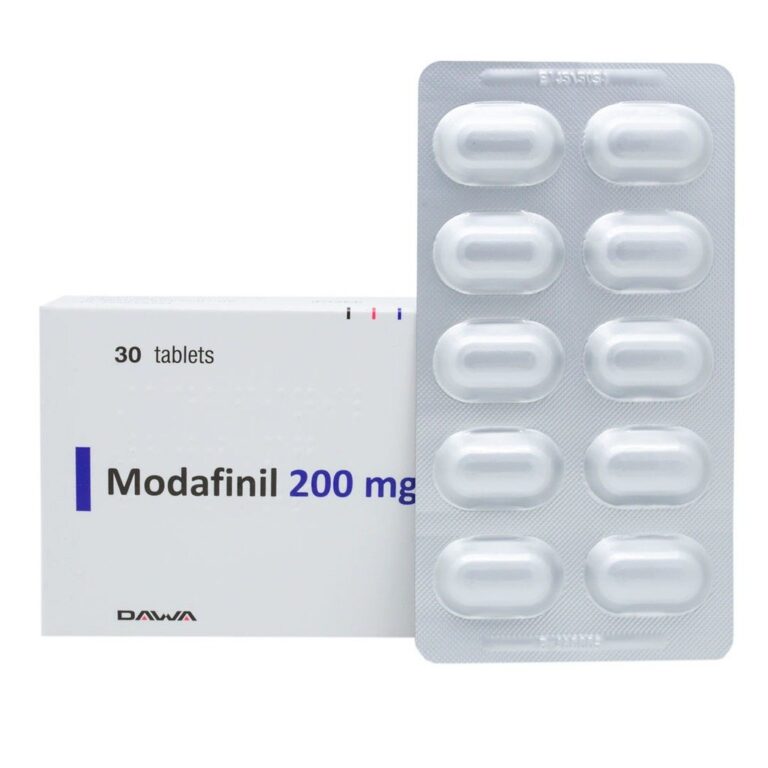
Dosage and Administration
The FDA-approved dosing for modafinil in OSA-related sleepiness is:
- Standard dose: 200 mg orally once daily in the morning (FDA, 2015).
- Doses up to 400 mg daily have been used, but no consistent evidence shows additional efficacy beyond 200 mg (FDA, 2015; Schwartz et al., 2003).
- Dose titration should be individualized based on patient response and tolerability.
- Dose adjustments are recommended for patients with severe hepatic impairment or elderly patients (FDA, 2015).
Safety and Side Effects
Common adverse effects include headache, nausea, nervousness, anxiety, and insomnia (FDA, 2015). Rare but serious reactions such as Stevens-Johnson syndrome, multi-organ hypersensitivity, and psychiatric symptoms (e.g., mania, hallucinations) have been reported, requiring immediate discontinuation if suspected (FDA, 2015).
Cardiovascular monitoring is advised in patients with pre-existing heart disease, as modafinil may affect blood pressure and heart rate (FDA, 2015). Given these risks, patients should be closely monitored, especially during early treatment phases (FDA, 2015; Chapman et al., 2016).
Clinical Considerations
- Modafinil is not a replacement for CPAP or other primary apnea treatments but serves to manage residual sleepiness (Schwartz et al., 2003; FDA, 2015).
- Prior to initiation, clinicians should exclude other causes of sleepiness such as insufficient sleep, other sleep disorders, or medications (Schwartz et al., 2003).
- Long-term safety data are limited; thus, treatment decisions should be individualized and regularly re-evaluated (Chapman et al., 2016).
Conclusion
Modafinil is an effective adjunct therapy for excessive daytime sleepiness in obstructive sleep apnea, especially in patients with residual sleepiness despite CPAP. It improves wakefulness and cognitive function, but does not treat the apnea itself. Safe use requires careful patient selection, dose management, and monitoring by healthcare professionals.
References
- Chapman, J. L., Kempler, L., Chang, C. L., Williams, S. C., Sivam, S., Wong, K. K., Yee, B. J., Grunstein, R. R., & Marshall, N. S. (2014). Modafinil improves daytime sleepiness in patients with mild to moderate obstructive sleep apnoea not using standard treatments: A randomised placebo-controlled crossover trial. Thorax, 69(3), 274–279. https://doi.org/10.1136/thoraxjnl-2013-203796
- Chapman, J. L., Vakulin, A., Hedner, J., Yee, B. J., & Marshall, N. S. (2016). Modafinil/armodafinil in obstructive sleep apnoea: A systematic review and meta-analysis. European Respiratory Journal, 47(5), 1420–1428. https://doi.org/10.1183/13993003.01509-2015
- Greenblatt, K., & Adams, N. (2023). Modafinil. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK531476/
- Schwartz, J. R., Hirshkowitz, M., Erman, M. K., & Schmidt-Nowara, W. (2003). Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: A 12-week, open-label study. Chest, 124(6), 2192–2199. https://doi.org/10.1378/chest.124.6.2192
- U.S. Food and Drug Administration. (2015). PROVIGIL® (modafinil) tablets, for oral use, C-IV [prescribing information]. U.S. Department of Health and Human Services. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020717s037s038lbl.pdf